Mastering Hierarchical Architectures and Non-Equilibrium Materials
A central goal in chemistry is to synthesize functional materials to address the current energy, environmental, and health challenges. The achievement of this objective requires the management of hierarchical complexity and non-equilibrium dynamics within heterogeneous materials for optimal efficiency of chemical processes. My past research has led to several breakthroughs in these field of porous materials, adsorption, and catalysis.

I. Pore Engineering
Pore engineering (Chem 2020) is the design and synthesis of porous structures with desired pore environments and properties. It is essential for many energy-related industrial applications including separation, storage, and catalysis. Metal-organic frameworks (MOFs) are regarded as a series of crystalline porous structures that are highly tunable over their components and pore spaces. The presence of labile or reactive sites including labile coordination bonds and dynamic covalent bonds enables researchers to precisely tune the pore environments for guest recognition and capture. I developed many synthetic methodology, including linker thermolysis (JACS 2018), reinstallation (Matter 2019), imprinting (JACS 2019), migration (Nature Chem. 2020; Matter 2020), modular synthesis (ACS Cent. Sci. 2018) and programming (JACS 2019). These methods (Nature Protoc. 2022) are expected to provide guidance on the synthesis of increasingly complex functional materials for a variety of practical applications.

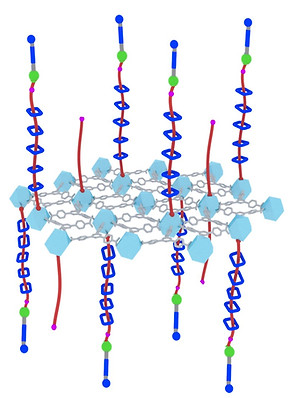
II. Active Adsorption
Numerous chemical processes, ranging from carbon capture and water remediation to catalysis and electrochemistry, involve the sorption of small molecules onto surfaces. However, over the past century, adsorption has been investigated extensively only in equilibrium systems, with a focus on the van der Waals interactions associated with physisorption and electronic interactions in the case of chemisorption. I discovered the first fundamentally new mode of adsorption—mechanisorption (Science 2021)—since the observation of physisorption and chemisorption in the 1930s, which results from non-equilibrium pumping to form mechanical bonds between adsorbents and adsorbates. Analogous to the mechanism in living organisms to control the active transport of ions across membranes, adsorbates are transported from one well-defined compartment—the bulk—to another well-defined compartment—the interface—thereby creating a very large chemical potential gradient commensurate with storing energy in a metastable state. This new non-equilibrium mechanisorption has wide implications (Nat. Rev. Chem. 2022) for future applications in the contexts of molecular recognition, optoelectronics, drug delivery, carbon capture, and water desalination. Mechanisorption extends, in a fundamental manner, the scope and potential of adsorption phenomena and offers a transformative approach to control chemistry at surfaces and interfaces. (Read More)
III. Heterogeneous Catalysis
Complex porous materials with hierarchical structures (Trends Chem. 2020) are expected to demonstrate unusual properties and behaviors. In particular, improving the ability to tune these hierarchical structures on multiple scales will be necessary for the advancement of cooperative catalysis, as it requires optimization of both the activity of the catalytic center and selectivity of the porous framework. The strategies I developed greatly enrich the scope and applicability of porous catalysts and offer opportunities to introduce variates and sequences into three dimensional hierarchical and cooperative systems (Chem 2020) for heterogeneous catalysis. Hierarchically porous MOFs with tailored pore environment and functionalities were used (ACS Catal. 2019) to heterogeneous catalysis such as the photochemical semisynthesis of an essential antimalarial drug, artemisinin (Adv. Sci. 2020).

Recent Publications



